We all have encountered the word “catalysis” during our high school chemistry. Catalyst is a substance which can accelerate the kinetics of a reaction without getting chemically involved in it. Ultimately, first question which arises in our mind is “ How does this happen?” In order to understand the behavior of catalyst, let me take you in the world of catalysis beyond the “high school definition”. In the figure below we can see the major discoveries in the field of catalysis before 1900.
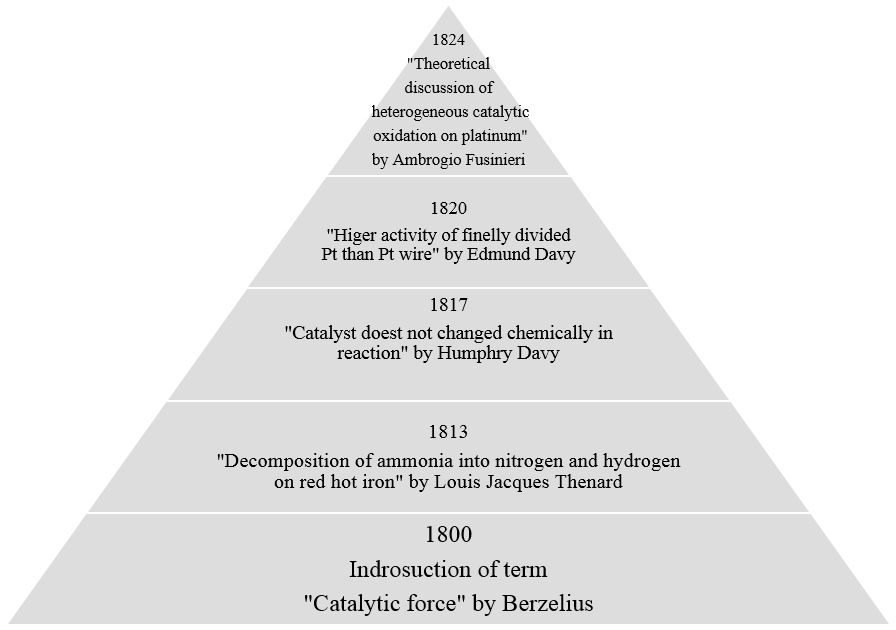
Figure 1: Chronological representation of the major research works in the field of catalysis.
A catalyst doesn’t contributes any energy to a chemical system as well as can’t change the position of the equilibrium. It can only alter the rate at which it is reached. Rate of reaction is directly proportional to activation energy (Arrhenius equation). This implies that a catalyst is providing a new pathway with lower activation energy which is not the case without catalytic reaction. The role of a catalyst is vividly illustrated in Figure 2; the green color arm represents the catalyst which helps the reactant and product come closer for reaction.
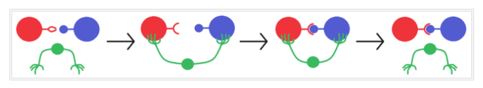
Figure 2: Graphical representation of catalytic reaction
(source: greenchemuoft.wordpress.com)
Catalysis is classified into two categories namely homogeneous and heterogeneous as shown in Figure 3. In homogeneous catalysis, the catalyst and reagent are in same phase. On the other hand in heterogeneous catalysis, catalyst and reagent are in different phase. Heterogeneous catalyst has more industrial importance as compared to homogeneous catalyst owing to its eco-friendliness, clean reaction as well as re-usability. Development and better understanding of heterogeneous catalysis has been identified as a prime research area by several research groups as it can help in addressing the challenges faced in the development of sustainable energy. The prime motive of research in this field is to find suitable catalyst which can efficiently transform energy form one form to another form. Surface chemistry is ameliorating in development of such a catalyst.
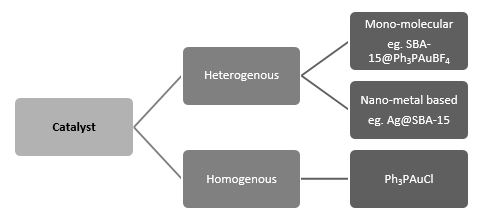
Figure 3: Classification of catalyst
Adsorption energy and activation energy is an important parameter in catalysis and the d-model in surface chemistry mainly deals with these two parameters. The d-model theory which describes the interaction between valence states and s and d states of transition metals and providing chemical reactivity on the transition metals surface in heterogeneous catalysis1. Surface chemistry is become more important when we are dealing the nano-metal catalyze reaction. Nano-metal catalyze reactions are important branch of heterogeneous catalysis due to higher reactivity and unusual behavior than bulk metal. Recently a team from the Lawrence Berkeley National Laboratory in the US and the Hebrew University in the US and the Hebrew University of Jerusalem in Israel have shown that structural defect and jagged surface at the edge of platinum and gold particles at the nanoscale are key hot spot for chemical reactivity2. This discovery drives surface chemists to the next level of research because now we can discern the active center in nano-metal catalyst. According to F. Dean Toste:
“We can now directly identify the important role of surface defect in activating industrially relevant catalytic processes.”
Researcher are seeking to develop economical and optimal method for designing new catalysts for our requirements. In the near future we will have better understanding of uncovered mechanisms of catalysts.
- Abild-pedersen, F.; Studt, F.; Bligaard, T. 2011, 108 (3), 937–943.
- Wu, C. Y.; Wolf, W. J.; Levartovsky, Y.; Bechtel, H. A.; Martin, M. C.; Toste, F. D.; Gross, E. Nature2017, 541 (7638), 511–515.
